MDD To MDR Gap Analysis Template PowerPoint & Google Slides
MDD to MDR Gap Analysis Presentation Slide
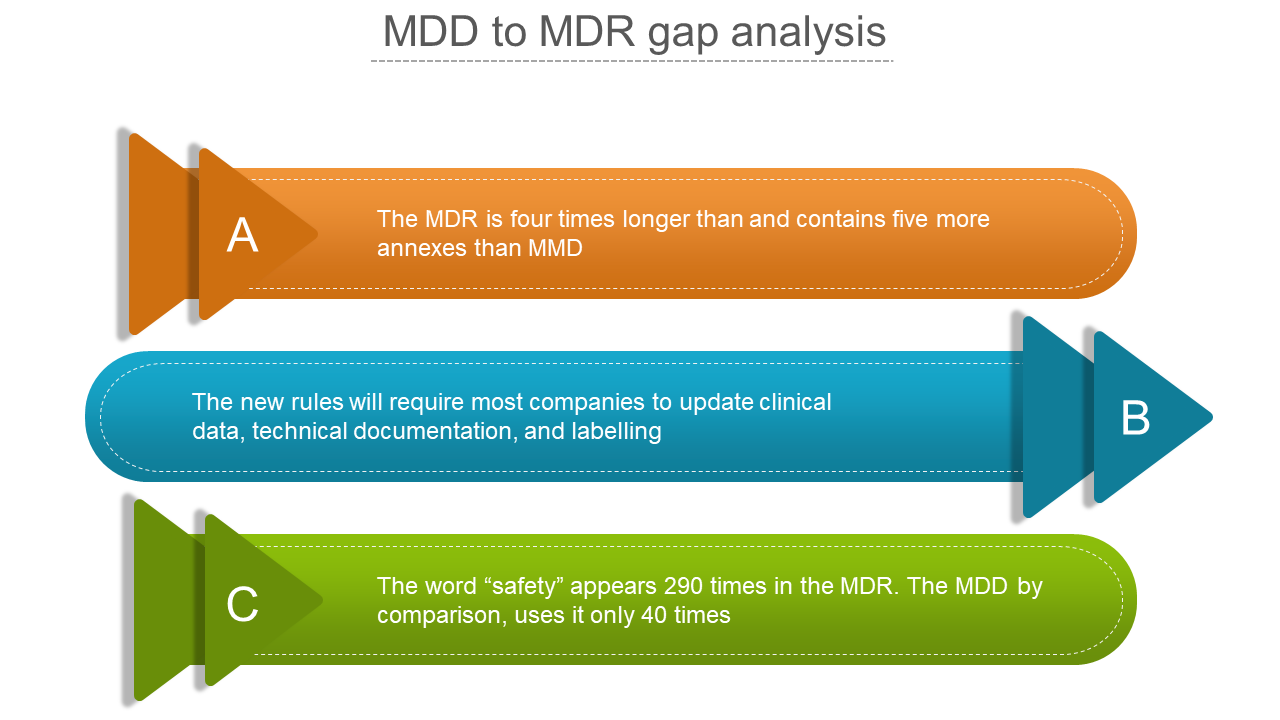
Are you seeking a comprehensive guide for a smooth transition? The MDD to MDR gap analysis process helps identify differences between the requirements of MDD and the new Medical Devices Regulation (MDR) in the European Union. Our customizable template features three arrows, each representing a crucial topic in the transition process, making it a valuable asset to your toolkit. The MDD and MDR are regulatory frameworks governing the safety and efficacy of medical devices sold in the EU. Let our template help guide you through the transition and ensure compliance with MDR.
Features of the template
- 100% customizable slides and easy to download.
- Slides are available in different nodes & colors.
- The slide contains 16:9 and 4:3 formats.
- Easy to change the colors of the slide quickly.
- Well-crafted template with an instant download facility.
- Highly compatible with PowerPoint and Google Slides.
- The perfect template for presenting your gap analysis report.
- Arrows makes your points clear to understand.
You May Also Like These PowerPoint Templates
Free